First, reveal the molecular mechanism of NLRC4 protein self-inhibition (Molecular Mechanisms)
On June 14, the research group of Professor Chai Jijie from the School of Life Sciences of Tsinghua University published a research paper titled "Crystal structure of NLRC4 reveals its autoinhibition mechanism" online in Science, which was reported for the first time. The crystal structure of the mouse NOD-like receptor NLRC4 in self-inhibitory state, and through structural analysis and biochemical experiments revealed the molecular mechanism of the protein to maintain self-inhibition (Molecular Mechanisms), which is also the first in the NOD-like receptor family to be resolved The nearly full-length protein crystal structure.
NOD-like receptors are a type of pattern recognition receptors found in the cytoplasm in recent years, capable of recognizing pathogenic molecules that enter the cell to elicit an immune response, and are an important part of the body's natural immune system. The abnormality of NOD-like receptors is closely related to many diseases, including various autoimmune diseases such as arthritis, various metabolic syndromes such as obesity, inflammatory bowel disease and the occurrence of tumors. The study of the mechanism of action of this family of proteins is becoming an important hotspot in the field of basic immunology.
The family of NOD-like receptors identified so far contains at least 22 human members and 34 murine members. NLRC4 is a member of the NOD-like receptor family and mainly recognizes bacterial flagellin and components of the type III secretion system. Under normal circumstances, NLRC4 is at rest by self-inhibition; when pathogen components enter the cell, it is recognized by NLRC4 to activate it; activated NLRC4 self-multiplexes, forming inflammatory bodies, producing a series of immune responses reaction. It is unclear how NLRC4 and other proteins in this family maintain self-inhibition, how to recognize ligands, and how to activate them.
The research group led by Professor Chai Jijie has always focused on the structure and function of the NOD-like receptor family. The phase of mouse NLRC4 protein crystal was successfully resolved using Shanghai Light Source Biomacromolecule Crystalline Station (BL17U1). The final crystal structure resolution is 3.2 ?, the structure shows that the single NLRC4 protein is in a self-inhibitory state in the form of monomer. The nucleotide-binding domain (NBD) in this protein binds to ADP, and the interaction between NDP and the winged-helix domain (WHD) mediated by ADP is important for maintaining NLRC4. The self-suppression state is critical. Helical domain 2 (HD2) also participates in maintaining the self-suppressed state of NLRC4 by interacting with a functionally important alpha helix in NBD. The leucine-rich repeat (LRR) domain at the C-terminus is just at the position where the protein multimerizes, thus further ensuring the maintenance of the self-inhibitory state. When the interaction between NBD-WHD, NBD-HD2 or NBD-LRR is broken by amino acid mutation, these mutants constitutively activate NLRC4 in the cell. The above structure and biochemical results indicate that the NLRC4 protein maintains a self-inhibitory state in a coordinated manner with multiple domains centered on NBD. The disclosure of the molecular mechanisms of NLRC4 protein self-inhibition not only deepened the understanding of the resting mechanism of this family of proteins, but also provided important clues for understanding the causes of abnormal activation of some disease-related mutants.
The picture shows the crystal structure of the mouse NOD-like receptor NLRC4 self-inhibited state
2. Significant progress has been made in the study of the mechanism of new coronavirus invasion into host cells
On July 7th, the Gaofu research group of the Institute of Microbiology, Chinese Academy of Sciences published a research paper titled Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26 online in the journal Nature, reporting their invasion of new coronavirus Significant progress has been made in the study of host cell mechanisms.
The new coronavirus, also known as the Middle East Respiratory Syndrome coronavirus (MERS-CoV), is another highly pathogenic coronavirus after SARS-CoV. Similar to SARS, patients with MERS-CoV infection may have fever, cough and acute respiratory distress syndrome, sometimes accompanied by renal failure, and the mortality rate is very high. Since the first report in September 2012, as of July 5, 2013, a total of 79 cases of MERS-CoV virus infection laboratory-confirmed cases have been notified to WHO worldwide, of which 42 have died. To date, laboratory confirmed cases have been reported in Jordan, Qatar, Saudi Arabia, the United Arab Emirates, France, Germany, Italy, Tunisia and the United Kingdom; limited local transmission has also occurred in France, Italy, Tunisia and the United Kingdom. For a while, MERS-CoV's high case fatality rate, human-to-human ability, and the trend of spreading to other countries have attracted attention and concern from all parties. Therefore, it is very important to understand the mechanism of MERS-CoV invasion into host cells.
MERS-CoV and SARS-CoV belong to the Coronaviridae family, Beta Coronavirus is a positive-strand RNA virus with a capsule. The virus envelope contains spike proteins (spike, S), which mediate the binding of the virus to host-specific receptor molecules, and is the most important molecule to initiate viral infection. Recent studies have shown that dipeptidyl peptidase-4 (DPPeptidyl peptidase 4, DPPIV / CD26) is a receptor for MERS-CoV in host cells. Identifying the binding mode of MERS-CoV S protein and CD26 is of great significance for the study of the MERS-CoV virus invasion mechanism and the discovery of effective drug targets. The Gaofu research group has long been devoted to the study of the transspecies transmission mechanism and immune molecular recognition of enveloped viruses. For the newly discovered virus receptor molecules, it has rapidly carried out functional studies on the structure and interaction of the complex.
The researchers first identified the part of the S protein responsible for binding to CD26, that is, the receptor binding domain (MERS-RBD); then successfully prepared high-purity MERS-RBD and protein complexes with CD26, And obtained high-quality crystals. The molecular structure of MERS-RBD monomer and ligand / receptor complex was finally analyzed using Shanghai Light Source Biomacromolecule Crystalline Station (BL17U1). MERS-RBD is composed of a core region and an external receptor recognition region (see Figure a). The structure of the core region is homologous to the spike molecules of SARS-CoV, while the external receptor recognition region appears as a unique composition of β-sheets. The structural unit recognizes the IV and V paddles in the “β-propeller†-like structure of the CD26 molecule. CD26 belongs to the type II transmembrane protein and exists on the cell membrane as a dimer. MERS-RBD binds to the distal membrane end of CD26, forming a U-shaped molecular structure (Figure b). In the process of recognition of receptors by viral ligands, the hydrogen bonds formed by side chain groups interact with hydrophilic interactions such as salt bridges. The details of these interactions at the molecular level provide an important reference for the design of small molecule drugs that target viral invasion.
The potential threat of MERS-CoV to public health security urgently needs specific and highly effective anti-MERS-CoV virus drugs. It is one of the most effective anti-virus means to block the combination and intrusion of viruses and prevent them from happening. The research results of the combination mode of MERS-RBD and CD26 in the research group have important guiding significance for the drug design against MERS-CoV virus.
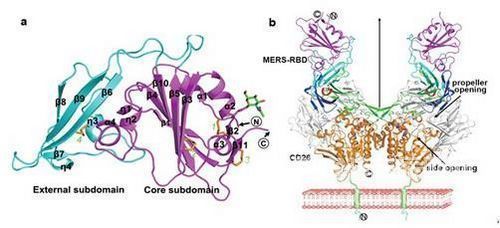
The picture shows the crystal structure of MERS-RBD and RBD / CD26 complex
Various products of DVD Cases, providing product images and basic parameters with each DVD Cases and DVD Cases; We are a 12 year professional Chinese manufacturer of DVD Cases, and look forward to your cooperation! my whatsapp is the 0086-13790846651
Long Single/double DVD case serie
dvd case,dvd box ,dvd cover
Shantou Yupeng Cdian Industry Co.LTD , https://www.headsetswireless.com