The UK ’s first stem cell human therapy trial led by Professor Peter Coffey of University College London has been approved by the British Medicines and Healthcare Products Authority (MHRA). This test is aimed at one of the most common eye diseases in the elderly in Western countries-macular degeneration of the retina.
Last week, the United States Jielong Biomedical Company announced that it would start the world's first human stem cell clinical trial of human embryos to treat spinal cord injury diseases. The U.S. Food and Drug Administration approved the trial and deemed it a major advance in stem cell research. This has also had a partial impact on the UK's approved trials.
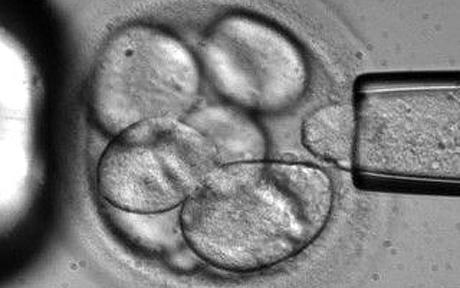
According to reports, embryonic stem cells are undifferentiated primary cells that appear a few days after conception, and have the ability to divide into any of the approximately 200 types of cells in the human body. However, they also share a common property with certain cancer cells-immortality, so implanting them directly in patients is very risky. In 2001, a hospital in New York implanted it into a patient ’s brain for treatment of Parkinson ’s disease, hoping that fetal tissue could form brain cells and produce dopamine. But as a result, the dopamine in the patient's brain was greatly reduced, the cell proliferation was out of control, and the patient's muscle twitching could not be controlled. Then Cornell University issued a warning: Stem cells also produce pre-cancer cells.
To ensure the safety of this trial, Kofi said that they will ensure that they only differentiate into normal cells before transplanting tissues to patients. Therefore, the possibility of stem cells becoming tumor factors no longer exists. Currently, he and the research team are using stem cells to grow cells that can form the retina. A "patch" containing cultured cells will be placed behind the retina to restore the patient's vision. "Once the test is started, it will soon be known whether it will work. The verification question is simple, whether the patient can see the light again." Kofi believes that for diseases such as macular degeneration of the retina, there is no need to treat the cells and patient tissue There is a complex interaction between them, so it is very likely to be the first cured case. If this method works, the treatment of diseases including liver disease, heart disease and diabetes will become simpler. However, the treatment of diseases such as Alzheimer's disease and Parkinson's disease that are difficult to accurately describe their environmental characteristics may require more effort in research. But no matter what, stem cells will eventually "change medicine."
Chris Masson, professor of regenerative medicine biotechnology at University College London, agrees. He said that before obtaining a safe and effective stem cell therapy, it will still undergo years of rigorous testing, and there may be obstacles and failures, but this human test marks the dawn of the "stem cell era".
Straight Knife Grinding Machine Model innovo-1 is a professional for sharpening variety of angled or straight blade sharpener fa
Straight Knife Grinding Machine Model innovo-1 is a professional for sharpening variety of angled or straight blade sharpener face. It has short grinding time, small amount of grinding, grinding and high precision, knife-edge sharp, reasonable price features.It is ideal for corporate small Printing, packaging, and individual users sharpening equipment
Knife Grinding Machine
Knife Grinding Machine, Blade Grinding Machine
Anhui Innovo Bochen Machinery Manufacturing Co., Ltd. , https://www.innovomachines.com